Date:
Midsemester Exam
Thermal Physics
Presence: 10p
Questions (3p each, 45p total):
- What do we mean by the multiplicity of a configuration?
- How the entropy of a configuration is related to the multiplicity?
- Name three extensive and three intensive thermodynamic parameters.
- What was our fundamental assumption regarding the probability of microstates for closed and isolated thermodynamic systems?
- What does the ergodic hypothesis state?
- What is the relation between the fundamental temperature and the absolute temperature (in the Kelvin scale)? Specify the physical quantities in the formula, and their SI units.
- Which law of thermodynamic is related to the energy conservation principle? Enounce this law.
- How do we mathematically characterize the fluctuations of a physical quantity?
- How could one calculate the partition function Z, using a sum over all energy values instead of a sum over all states?
- In the low and high temperature limit how does the specific heat of a solid body depend on temperature? What is the mathematical condition for the low temperature limit?
- What are the phonons? What is common and different between phonons and photons?
- What is Df in the Nyquist theorem?
- How many polarizations does an elastic wave have? How many polarizations does an electromagnetic wave have?
- Write up the thermodynamic identity (differential form of the internal energy) when we consider a process in which the entropy, volume and particle number can all change. Specify the physical quantities in this equation.
- Let us consider an open thermodynamic system S, with a chemical potential m in contact with a heat-bath at temperature t. How would one calculate the probability that S has N particles at a given time-moment? Specify the physical quantities in your equation.
Problems (45p total):
1. We consider a binary magnetic system (S) composed of six (6) non-interacting magnetic-moments (spins), inside an external magnetic field B. The magnetic moments of the individual spins are +/- m. (see the figure). The system is in contact with a heat-bath at temperature t.
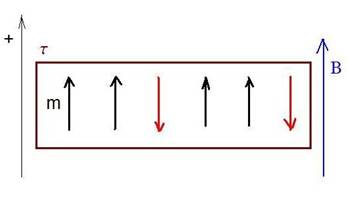
6 binary magnetic moments in an external magnetic field B, and in
contact with a heat-bath at temperature t.
a.) Calculate the probability that at a given time-moment the S system will be in the state specified in the figure? (5p)
b.)
Calculate the probability that at a given
time-moment the S system will have a total magnetization: +2m
? (5p)
c.) Calculate the average magnetization of the S system for a t=mB temperature. (5p)
d.) Calculate the average magnetization for a tฎฅ temperature. (5p)
2. We consider a
thermodynamic system composed of N particles in which the multiplicity of a
configuration with a given U internal energy is given as: g(U)=C
U7N/2. Show that the caloric equation of state for this
system is U=7Nt/2.
Specify a thermodynamic system, for which the above caloric equation of state is
valid! (10+5=15p)
3. Consider a system that may be unoccupied with energy zero or occupied by one particle in three possible states with energy 0, e and 2e (three level system). The system is in diffusive equilibrium with a heat-bath at temperature t. Assuming that the chemical potential of the particles is m, calculate the thermal average occupancy of the state with energy 2e. (10 p)
Total maximum: 100p (25% in final)
Total obtained: ..
Percent in the final grade:
..